Is the Pre-Shaping of an Orbital Implant on a Patient-Specific 3D-Printed Model Advantageous Compared to Conventional Free-Hand Shaping? A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Assessment of Risk of Bias
2.5. Data Synthesis
3. Results
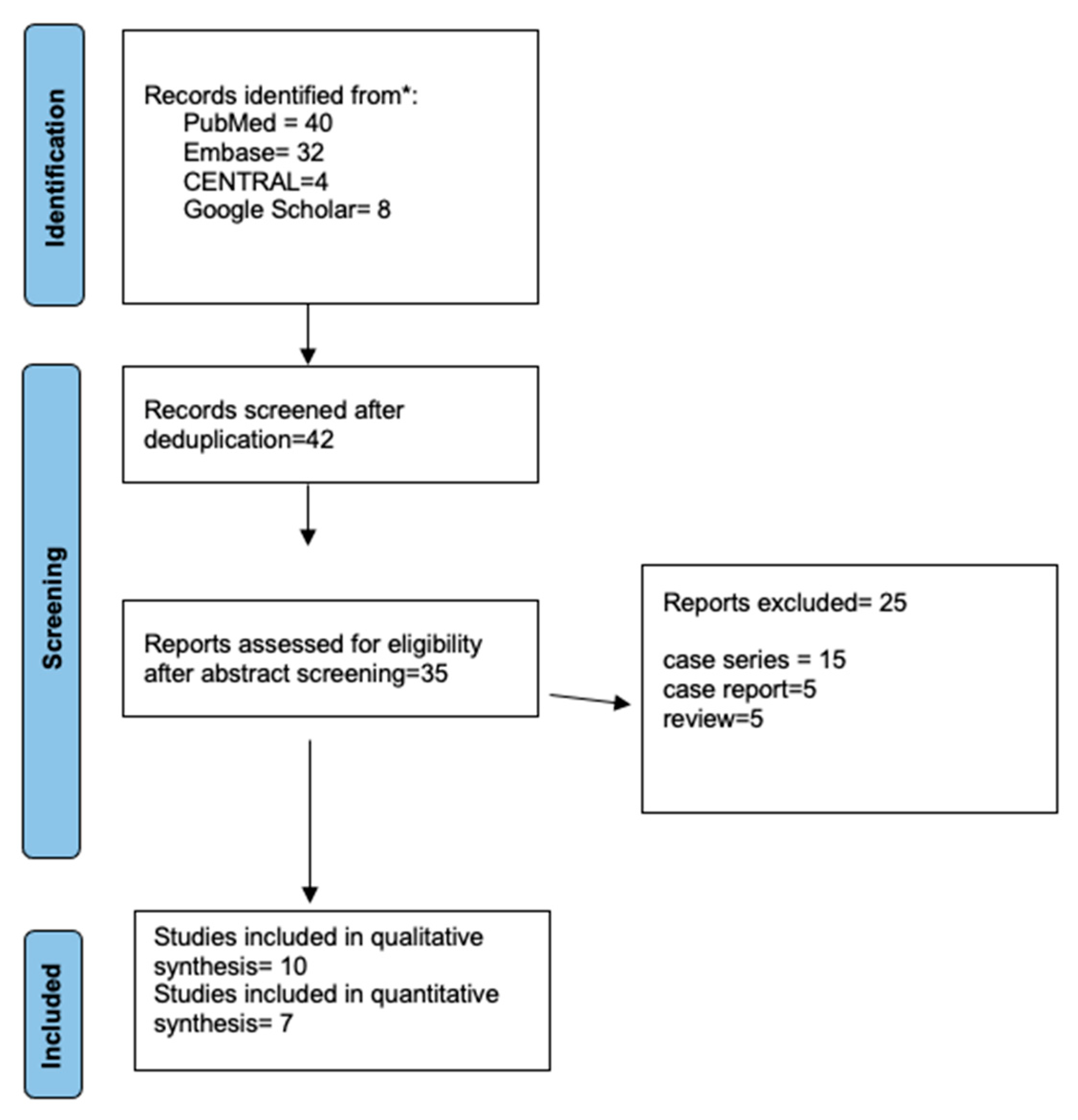
3.1. Search Results and Study Characteristics
3.2. Risk of Bias Assessment
3.3. Outcome and Meta-Analysis
3.3.1. Accuracy of Fit
3.3.2. Restoration of Orbital Defect and Volume
3.3.3. Correction of Orbital Dystopia
3.3.4. Complications
3.3.5. Operative Time
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Mast, G.; Ehrenfeld, M.; Cornelius, C.-P.; Tasman, A.-J.; Litschel, R. Maxillofacial Fractures: Midface and internal orbit-part II: Principles and surgical treatment. Facial Plast. Surg. 2015, 31, 357–367. [Google Scholar] [PubMed]
- Valencia, M.R.; Miyazaki, H.; Ito, M.; Nishimura, K.; Kakizaki, H.; Takahashi, Y. Radiological findings of orbital blowout fractures: A review. Orbit 2021, 40, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S. Primary Orbital Fracture Repair. Atlas Oral. Maxillofac. Surg. Clin. 2021, 29, 51–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.H.; Hwang, K. The Frequency of decreased visual acuity in orbital fractures. J. Craniofac. Surg. 2015, 26, 1581–1583. [Google Scholar] [CrossRef]
- Dubois, L.; Steenen, S.A.; Gooris, P.J.J.; Mourits, M.P.; Becking, A.G. Controversies in orbital reconstruction—II. Timing of post-traumatic orbital reconstruction: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 433–440. [Google Scholar] [CrossRef]
- Gart, M.S.; Gosain, A.K. Evidence-based medicine: Orbital floor fractures. Plast. Reconstr. Surg. 2014, 134, 1345–1355. [Google Scholar] [CrossRef]
- Bratton, E.M.; Durairaj, V.D. Orbital implants for fracture repair. Curr. Opin. Ophthalmol. 2011, 22, 400–406. [Google Scholar] [CrossRef]
- Seven, E.; Tellioglu, A.T.; Inozu, E.; Ozakpinar, H.R.; Horoz, U.; Eryilmaz, A.T.; Karamursel, S. Reconstruction of orbital floor with auricular concha. J. Craniofac. Surg. 2017, 28, e713–e717. [Google Scholar] [CrossRef]
- Düzgün, S.; Kayahan Sirkeci, B. Comparison of postoperative outcomes of graft materials used in reconstruction of blowout fractures. Turk. J. Trauma Emerg. Surg. 2020, 26, 538–544. [Google Scholar]
- Pereira, R.D.S.; Jorge-Boos, F.B.D.; Hochuli-Vieira, E.; da Rocha, H.V.J.; Homsi, N.; de Melo, W.M. Management of pure medial orbital wall fracture with autogenous bone graft. J. Craniofac. Surg. 2013, 24, e475–e477. [Google Scholar] [CrossRef]
- Baino, F. Biomaterials and implants for orbital floor repair. Acta Biomater. 2011, 7, 3248–3266. [Google Scholar] [CrossRef]
- Seen, S.; Young, S.; Lang, S.S.; Lim, T.-C.; Amrith, S.; Sundar, G. Orbital implants in orbital fracture reconstruction: A ten-year series. Craniomaxillofac. Trauma Reconstr. 2021, 14, 56–63. [Google Scholar] [CrossRef]
- Saha, A.K.; Samaddar, S.; Kumar, A.; Chakraborty, A.; Deb, B. A Comparative Study of Orbital Blow Out Fracture Repair, Using Autogenous Bone Graft and Alloplastic Materials. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 542–549. [Google Scholar] [CrossRef]
- Bly, R.A.; Chang, S.-H.; Cudejkova, M.; Liu, J.J.; Moe, K.S. Computer-guided orbital reconstruction to improve outcomes. JAMA Facial Plast. Surg. 2013, 15, 113–120. [Google Scholar] [CrossRef]
- Jansen, J.; Schreurs, R.; Dubois, L.; Maal, T.J.J.; Gooris, P.J.J.; Becking, A.G. Intraoperative imaging in orbital reconstruction: How does it affect the position of the implant? Br. J. Oral. Maxillofac. Surg. 2020, 58, 801–806. [Google Scholar] [CrossRef]
- Schlittler, F.; Vig, N.; Burkhard, J.P.; Lieger, O.; Michel, C.; Holmes, S. What are the limitations of the non-patient-specific implant in titanium reconstruction of the orbit? Br. J. Oral. Maxillofac. Surg. 2020, 58, e80–e85. [Google Scholar] [CrossRef]
- Ordon, A.J.; Kozakiewicz, M.; Wilczynski, M.; Loba, P. The influence of concomitant medial wall fracture on the results of orbital floor reconstruction. J. Craniomaxillofac. Surg. 2018, 46, 573–577. [Google Scholar] [CrossRef]
- Schlittler, F.; Schmidli, A.; Wagner, F.; Michel, C.; Mottini, M.; Lieger, O. What is the incidence of implant malpositioning and revision surgery after orbital repair? J. Oral. Maxillofac. Surg. 2018, 76, 146–153. [Google Scholar] [CrossRef]
- Nikunen, M.; Rajantie, H.; Marttila, E.; Snäll, J. Implant malposition and revision surgery in primary orbital fracture reconstructions. J. Craniomaxillofac. Surg. 2021, 49, 837–844. [Google Scholar] [CrossRef]
- Diment, L.E.; Thompson, M.S.; Bergmann, J.H.M. Clinical efficacy and effectiveness of 3D printing: A systematic review. BMJ Open 2017, 7, e016891. [Google Scholar] [CrossRef]
- Meglioli, M.; Naveau, A.; Macaluso, G.M.; Catros, S. 3D printed bone models in oral and cranio-maxillofacial surgery: A systematic review. 3D Print Med. 2020, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.F.; Evans, P.L.; Bocca, A.; Patton, D.W.; Sugar, A.W.; Baxter, P.W. Customized titanium reconstruction of post-traumatic orbital wall defects: A review of 22 cases. Int. J. Oral. Maxillofac. Surg. 2011, 40, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Probst, F.A.; Cornelius, C.P.; Otto, S.; Malenova, Y.; Probst, M.; Liokatis, P.; Haidari, S. Accuracy of free-hand positioned patient specific implants (PSI) in primary reconstruction after inferior and/or medial orbital wall fractures. Comput. Biol. Med. 2021, 137, 104791. [Google Scholar] [CrossRef] [PubMed]
- Kormi, E.; Männistö, V.; Lusila, N.; Naukkarinen, H.; Suojanen, J. Accuracy of patient-specific meshes as a reconstruction of orbital floor blowout fractures. J. Craniofac. Surg. 2021, 32, e116–e119. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Online Meta. Available online: https://smuonco.shinyapps.io/Onlinemeta/ (accessed on 1 August 2022).
- Kozakiewicz, M.; Elgalal, M.; Piotr, L.; Broniarczyk-Loba, A.; Stefanczyk, L. Treatment with individual orbital wall implants in humans—1-Year ophthalmologic evaluation. J. Craniomaxillofac. Surg. 2011, 39, 30–36. [Google Scholar] [CrossRef]
- Zimmerer, R.M.; Ellis, E., III; Aniceto, G.S.; Schramm, A.; Wagner, M.E.; Grant, M.P.; Cornelius, C.P.; Strong, E.B.; Rana, M.; Chye, L.T.; et al. A prospective multicenter study to compare the precision of post-traumatic internal orbital reconstruction with standard preformed and individualized orbital implants. J. Craniomaxillofac. Surg. 2016, 44, 1485–1497. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jeong, W.S.; Park, T.K.; Choi, J.W.; Koh, K.S.; Oh, T.S. The accuracy of patient specific implant prebented with 3D-printed rapid prototype model for orbital wall reconstruction. J. Craniomaxillofac. Surg. 2017, 45, 928–936. [Google Scholar] [CrossRef]
- Fan, B.; Chen, H.; Sun, Y.-J.; Wang, B.-F.; Che, L.; Liu, S.-Y.; Li, G.-Y. Clinical effects of 3-D printing-assisted personalized reconstructive surgery for blowout orbital fractures. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2051–2057. [Google Scholar] [CrossRef]
- Raisian, S.; Fallahi, H.R.; Khiabani, K.S.; Heidarizadeh, M.; Azdoo, S. Customized titanium mesh based on the 3D printed model vs. manual intraoperative bending of titanium mesh for reconstructing of orbital bone fracture: A randomized clinical trial. Rev. Recent Clin. Trials. 2017, 12, 154–158. [Google Scholar] [CrossRef]
- Zieliński, R.; Malińska, M.; Kozakiewicz, M. Classical versus custom orbital wall reconstruction: Selected factors regarding surgery and hospitalization. J. Craniomaxillofac. Surg. 2017, 45, 710–715. [Google Scholar] [CrossRef]
- von Wilmowsky, C.; Schwertner, M.G.; Nkenke, E.; Moest, T.; Adler, W.; Ebker, T. Use of CAD-based pre-bent implants reduces theatre time in orbital floor reconstruction: Results of a prospective study. Br. J. Oral. Maxillofac. Surg. 2020, 58, 753–758. [Google Scholar] [CrossRef]
- Sigron, G.R.; Rüedi, N.; Chammartin, F.; Meyer, S.; Msallem, B.; Kunz, C.; Thieringer, F.M. Three-dimensional analysis of isolated orbital floor fractures pre- and post-reconstruction with standard titanium meshes and “hybrid” patient-specific implants. J. Clin. Med. 2020, 9, 1579. [Google Scholar] [CrossRef]
- Sigron, G.R.; Barba, M.; Msallem, B.; Berg, B. Functional and cosmetic outcome after reconstruction of isolated, unilateral orbital floor fractures (blowout fractures) with and without the support of 3D-printed orbital anatomical models. J. Clin. Med. 2021, 10, 3509. [Google Scholar] [CrossRef]
- Gupta, S.; Mehrotra, D.; Singh, P.K.; Vignesh, U.; Bhave, S.; Katrolia, R. Quality of life after reconstruction of traumatic orbital floor defects using titanium mesh and medpore: A randomised controlled trial. J. Oral. Biol. Craniofac. Res. 2021, 11, 200–203. [Google Scholar] [CrossRef]
- Murray-Douglass, A.; Snoswell, C.; Winter, C.; Harris, R. Three-dimensional (3D) printing for post-traumatic orbital reconstruction, a systematic review and meta-analysis. Br. J. Oral. Maxillofac. Surg. 2022, 60, 1176–1183. [Google Scholar] [CrossRef]
- Bartoli, D.; Fadda, M.T.; Battisti, A.; Cassoni, A.; Pagnoni, M.; Riccardi, E.; Sanzi, M.; Valentini, V. Retrospective analysis of 301 patients with orbital floor fracture. J. Cranio-Maxillofac. Surg. 2015, 43, 244–247. [Google Scholar] [CrossRef]
- Sozzi, D.; Gibelli, D.; Canzi, G.; Tagliaferri, A.; Monticelli, L.; Cappella, A.; Bozzetti, A.; Sforza, C. Assessing the precision of post-traumatic orbital reconstruction through “mirror” orbital superimposition: A novel approach for testing the anatomical accuracy. J. Craniomaxillofac. Surg. 2018, 46, 1258–1262. [Google Scholar] [CrossRef]
- Moon, S.J.; Lee, W.J.; Roh, T.S.; Baek, W. Sex-related and racial variations in orbital floor anatomy. Arch. Craniofac. Surg. 2020, 21, 219. [Google Scholar] [CrossRef]
- Nilsson, J.; Nysjö, J.; Carlsson, A.P.; Thor, A. Comparison analysis of orbital shape and volume in unilateral fractured orbits. J. Craniomaxillofac. Surg. 2018, 46, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.P.; Mayer, P.; Ehrenfeld, M.; Metzger, M.C. The Orbits—Anatomical features in view of innovative surgical methods. Facial Plast. Surg. 2014, 30, 487–508. [Google Scholar] [PubMed]
- Gooris, P.J.J.; Muller, B.S.; Dubois, L.; Bergsma, J.E.; Mensink, G.; van den Ham, M.F.E.; Becking, A.G.; Seubring, K. Finding the Ledge: Sagittal Analysis of Bony Landmarks of the Orbit. J. Oral. Maxillofac. Surg. 2017, 75, 2613–2627. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kang, D.H.; Gu, J.H. The Correlation between the Orbital Volume Ratio and Enophthalmos in Unoperated Blowout Fractures. Arch. Plast. Surg. 2016, 43, 518. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; He, Y.; An, J.; Zwahlen, R.A. Correlation between volume of herniated orbital contents and the amount of enophthalmos in orbital floor and wall fractures. J. Oral. Maxillofac. Surg. 2012, 70, 68–73. [Google Scholar] [CrossRef]
- Brucoli, M.; Arcuri, F.; Cavenaghi, R.; Benech, A. Analysis of complications after surgical repair of orbital fractures. J. Craniofac. Surg. 2011, 22, 1387–1390. [Google Scholar] [CrossRef]
- Boyette, J.R.; Pemberton, J.D.; Bonilla-Velez, J. Management of orbital fractures: Challenges and solutions. Clin. Ophthalmol. 2015, 9, 2127–2137. [Google Scholar] [CrossRef]
- Abbate, V.; Iaconetta, G.; Califano, L.; Pansini, A.; Bonavolontà, P.; Romano, A.; Orabona, G.D.A. Self-Made Rapid Prototyping Technique for Orbital Floor Reconstruction: Showcases for Technical Description. J. Craniofac. Surg. 2019, 30, 2106–2110. [Google Scholar] [CrossRef]

| Acronym Definition | Description |
|---|---|
| (P) Population | Patients of Any Age with Post-Traumatic Orbital Wall Defect That Required Reconstruction |
| (I) Intervention | Pre-shaped reconstructive implant on an anatomic 3D-printed model of the orbital wall defect |
| (C) Control | Conventional free-hand intraoperative-shaped implant |
| (O) Outcomes | Primary outcome: Fit of the implant, correction of orbital volume and globe position compared to the contralateral uninjured orbit Secondary outcomes: Operative time, complications, the cost difference |
| (S) Study design | Studies in humans, including randomized control trials (RCTs). Uncontrolled clinical trials and prospective or retrospective comparative studies |
| Author/Year | Study Sample | Country | Study Type | Inclusion Criteria | Follow-Up and Attrition (n) |
|---|---|---|---|---|---|
| Kozakiewicz 2011 [28] | MFS = 12 3DP = 12 | Poland | Retrospective | Orbital fractures without any coexisting central nervous system or globe injury | 12 months, n = 24 |
| Zimmerer 2016 [29] | MFS = 95 3DP = 100 | Germany, USA, Spain, Singapore, Austria | Prospective Controlled Multicenter trial | Patients > 18 years with fracture of the orbital floor and/or medial wall not older than 21 days | 12 weeks, n = 145 |
| Kim 2017 [30] | MFS = 38 3DP = 44 | Korea | Retrospective | Isolated blowout fracture lying unilaterally in the medial or inferior orbit within 1 month from the occurrence | 6 months, n = 82 |
| Fan 2017 [31] | MFS = 27 3DP = 29 | China | Retrospective | NG | NG |
| Raisian 2017 [32] | MFS = 5 3DP = 5 | Iran | RCT | Orbital bone fracture with at least 2 mm enophthalmos or vertical dystopia or diplopia | 4 months, n = 10 |
| Zielinski 2017 [33] | MFS = 54 3DP = 16 | Poland | Retrospective | Unilateral side lesion due to trauma, neoplasm, or orbital decompression | NG |
| Wilmosky 2020 [34] | MFS = 11 3DP = 25 | Germany | Prospective | Unilateral fractures of the orbital floor with a large defect requiring mesh | 6 months |
| Sigron 2020 [35] | MFS = 12 3DP = 10 | Switzerland | Retrospective | Unilateral isolated orbital wall fracture | NG |
| Sigron 2021 [36] | MFS = 13 3DP = 17 | Switzerland | Retrospective | Unilateral isolated orbital wall fracture requiring surgery with orbital floor mesh | NG |
| Gupta 2021 [37] | MFS = 16 3DP = 23 | India | RCT | Orbital floor fracture with diplopia, enophthalmos, paraesthesia, or a post-traumatic residual deformity | 6 months |
| Study | Accuracy of Fit | Defect Area and Volume | Correction of Orbital Dystopia | Operative Time (min) | Complications |
|---|---|---|---|---|---|
| Kozakiewicz 2011 [28] | ER BSV loss (p = 0.021) * Reduction in double vision area (p = 0.015) Improved primary globe position correction (p = 0.012) * | ||||
| Zimmerer 2016 [29] | Variance of differences in orbital volume non-CAD based = 0.9 mL2 CAD-based = 0.3 mL2; (p < 0.001) | Sagittal globe position: (p = 0.079) Pupillary height: NSD Diplopia: NSD 80% of CAD-based implants inserted by a less experienced surgeon (<10 years of experience) Sensory disturbance: NSD | MFS: 71; 3DP: 60 | ||
| Kim 2017 [30] | Medial wall fracture Layout angle (°) MFS: 9.03 ± 4.9; 3DP: 3.49 ± 1.97 (p < 0.001 *) Inferior wall fractures (°) MFS: 4.69 ± 2.51; 3DP: 2.23 ± 1.37; (p < 0.001 *) Gap Length Medial wall: SD * Inferior wall: NSD | Reduction in the Bone defect area Medial wall fracture MFS: 18.7 ± 15.41; 3DP: 8.03 ± 3.5 (p < 0.01) Inferior wall fracture MFS: 12.8 ± 4.92; 3DP: 7.14 ± 5.74 (p < 0.01) | MFS: 77.7; 3DP: 75.6 (p = 0.519) | Revision surgery for enophthalmos and restricted motility MFS: 2; 3DP: 0 Residual diplopia > 6 months MFS = 2; 3DP = 0 | |
| Fan 2017 [31] | DMW 5.60 ± 0.90 mm; 2.51 ± 0.53 mm (SD) DMD 4.61 ± 0.89 mm; 2.58 ± 0.46 mm (SD) DAR 84.05 ± 20.89 mm2; 43.59 ± 9.53 mm2 (SD) DAG 12.58 ± 5.04°; 2.82 ± 0.44° (SD) | Postoperative Enophthalmos MFS: 2.5 ± 1.0 mm; 3DP: 1.0 ± 0.5 mm (p < 0.05 *) | MFS: 95.37 ± 22.19; 3DP: 75.34 ± 15.68 (p < 0.05 *) | ||
| Raisian 2017 [32] | A significant difference in mean postoperative enophthalmos between the two groups after surgery (p < 0.01 *) at 1 week, 1 month, and 4 months, respectively | ||||
| Zielinski 2017 [33] | Shorter surgery time in patients with individual implants | Higher intraoperative bleeding in patients treated with intraoperative bending titanium mesh (p < 0.01) * | |||
| Wilmosky 2020 [34] | Accuracy of the implant form of CAD-based pre-bent titanium meshes Accurate = 17 Too large = 6 Too small = 2 | MFS: 11.1 ± 7.7; 3DP: 5.5 ± 5.4 (p < 0.01 *) | |||
| Sigron 2020 [35] | Mean ± SD absolute volume difference between the conventional and interventional groups MFS: 1.6 ± 1.2 mL; 3DP: 1.0 ± 0.7 mL (p = 0.002 *) Fractured area MFS: 408.5 ± 137.5; 3DP: 389.4 ± 135.1 (NSD) Maximum fracture collapse MFS: 6.9 ± 2.3 mm; 3DP: 8.6 ± 5.4 (NSD) | MFS: 99.8 ± 28.9; 3DP: 57.3 ± 23.4 (p = 0.001 *) | Postoperative length of hospital stay MFS: 4.6 (3.9) days 3DP: 3.8 (3.0) days (NSD) | ||
| Sigron 2021 [36] | With “hybrid” patient-specific titanium meshes, the functional and cosmetic outcome (diplopia, enophthalmos, ocular motility, and sensory disturbance) improved: NSD | MFS: 94.8 ± 33.0; 3DP: 58.9 ± 20.1 (p = 0.003 *) | |||
| Gupta 2021 [37] | Success score with material Pre-shaped titanium mesh: 9.26 ± 1.29; Biopore™: 8.25 ± 1.65 (p = 0.049) Positive correlation between Success score & QOL score (p = 0.034 *) Enophthalmos correction success score for pre-shaped titanium mesh: 10; Biopore™: 8.75 (p = 0.028 *) Hypoglobus correction success score for pre-shaped titanium mesh: 9; Biopore™: 7.50 (p = 0.047 *) Diplopia correction success score for pre-shaped titanium mesh: 9.78; Biopore™: 9.06; (p = 0.200) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.K.; Khanal, N.; Chaulagain, R.; Sharma, N.; Thieringer, F.M. Is the Pre-Shaping of an Orbital Implant on a Patient-Specific 3D-Printed Model Advantageous Compared to Conventional Free-Hand Shaping? A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3426. https://doi.org/10.3390/jcm12103426
Singh AK, Khanal N, Chaulagain R, Sharma N, Thieringer FM. Is the Pre-Shaping of an Orbital Implant on a Patient-Specific 3D-Printed Model Advantageous Compared to Conventional Free-Hand Shaping? A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(10):3426. https://doi.org/10.3390/jcm12103426
Chicago/Turabian StyleSingh, Ashutosh Kumar, Nikita Khanal, Rajib Chaulagain, Neha Sharma, and Florian M. Thieringer. 2023. "Is the Pre-Shaping of an Orbital Implant on a Patient-Specific 3D-Printed Model Advantageous Compared to Conventional Free-Hand Shaping? A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 10: 3426. https://doi.org/10.3390/jcm12103426
APA StyleSingh, A. K., Khanal, N., Chaulagain, R., Sharma, N., & Thieringer, F. M. (2023). Is the Pre-Shaping of an Orbital Implant on a Patient-Specific 3D-Printed Model Advantageous Compared to Conventional Free-Hand Shaping? A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(10), 3426. https://doi.org/10.3390/jcm12103426









